
Fossil and model reconstruction of the Late Triassic coelacanth, Diplurus newarki, commonly found in New Jersey and Pennsylvania. The model was designed by vertebrate paleontologist Bobb Schaeffer. Image credit: Yale Peabody Museum / Curious Sengi.
From fossil to the end of a fisherman’s line, the scientific discovery of a living coelacanth in December 1938 is a favorite story of resurrection, determination of a young curator — Marjorie Courtenay-Latimer (1907 – 2004) — at the East London Museum in South Africa, international political intrigue, and intense excitement over what this living (and largely unchanged) representative of an ancient lineage could reveal about the origins of tetrapods like us.

A living coelacanth, Latimeria chalumnae, generally indifferent to paparazzi and awed divers. Image credit: Projet Gombessa via X-Ray Mag.
Courtenay-Latimer famously describes her initial encounter with a coelacanth that was hauled into a local fish market:
I picked away at the layers of slime to reveal the most beautiful fish I had ever seen. It was five foot long, a pale mauvy blue with faint flecks of whitish spots; it had an iridescent silver-blue-green sheen all over. It was covered in hard scales, and it had four limb-like fins and a strange puppy dog tail. (Quoted from Amemiya, Dorrington, & Meyer 2014.)

Marjorie Courtenay-Latimer included this sketch in a letter sent to J.L.B. Smith, requesting his help in identifying an unusual fish brought in as by-catch on a local fishing vessel. Note that Courtenay-Latimer drew the tail (labeled “caudal fin” here) twice to bring attention to that distinctive feature. Image credit: African Coelacanth Ecosystem Programme (ACEP)/ South African Institute for Aquatic Biodiversity (SAIAB) via Twitter.
Uncertain what to make of this strange fish, Courtenay-Latimer writes to a scientific colleague and accomplished amateur ichthyologist, J.L.B. Smith (1897 – 1968). Her sketch of the mystery animal sealed the deal — Smith identified it as a coelacanth, a fish believed to have been extinct since the Late Cretaceous, about 70 million years ago (Amemiya et al. 2013). Key features to this thrilling recognition was the presence of muscular limb-like fins and the unusual “puppy dog tail” (Hissmann & Fricke 1996).

Courtenay-Latimer and the fish that shook the world. Confounded by the Christmas holidays and locals reluctant to have anything to do with this quickly decomposing, oily fish, this young curator managed to convince a taxidermist to salvage the skin. Courtenay-Latimer remained an active and beloved part of the coelacanth research community until her death at age 97. Image credit: Wikipedia.
The paired lobe fins were of great interest. There were four of them, each one filled with muscle and bone and attached to the central, axial skeleton by a rudimentary limb girdle. Smith made early speculations on the pattern of movement of these lobe fins (Fricke & Hissmann 1992). It was hypothesized that the fin strokes would be homologous to how animals walk on land and that the coelacanth was pushing itself along the ocean floor with its limb-like fins (Fricke et al. 1987; Fricke & Hissmann 1992; Forey 1998).
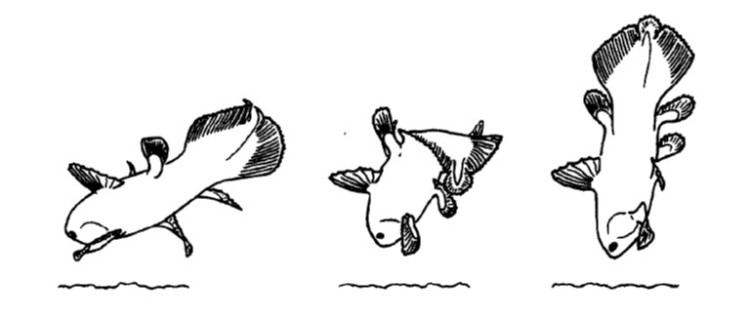
Observations of live animals in their deep water habitats showed that coelacanths do not trot around the sea bottom at all, but instead favor life as drifters in the current or generally remaining in the same place, only making the occasional fast start to change direction or lunge towards a tasty item (Fricke et al. 1987; Uyeno 1991; Forey 1998). While the lobe fins do move alternatively like legs, these are probably acting more as stabilizers. Most of the slow, forward movement is propelled by the second dorsal and first anal fins sculling in unison (Fricke et al. 1987; Uyeno 1991; Fricke & Hissmann 1992). This is very unlike many fish familiar to us that rely on their tails or the side-to-side lateral flexures of the body to generate forward thrust (Hissmann & Fricke 1996).

The coelacanth propels itself forward using its second dorsal and first anal fins, as marked by the arrows. These two fins beat in unison. This mode of swimming is very similar to that found in [the completely unrelated] Ocean Sunfish (Mola mola). The paired pectoral and pelvic fins serve more to stabilize the animal. Image credit: YouTube.

Detail of the epicaudal lobe of the coelacanth Latimeria menadoensis from Indonesia. Image credit: Seapics.com.

Skeleton of a modern coelacanth on display at the Naturhistorisches Museum in Vienna, Austria. Both the sensory lateral line and the notochord extend to the end of the epicaudal lobe. The notochord is a flexible dorsal rod that acts as a developmental and evolutionary precursor to the vertebral column; this structure is retained in adult coelacanths. Image credit: Fuck Yeah Coelacanths! on Tumblr.

An especially long epicaudal lobe preserved in the Late Triassic Diplurus sp. Image credit: Yale Peabody Museum / Curious Sengi.
In 1948, paleontologist Bobb Schaeffer (1913 – 2004) remarked that: “It is difficult to visualize a separate and distinct function for the supplementary caudal [i.e., epicaudal] in spite of its morphological discreteness.” And so it seems to stand. Thorough analysis of video footage by Hans Fricke and Karen Hissmann demonstrate that the epicaudal is not coordinated with the movement of any other fins. Neither does it move continuously. It just seems to carry on in its own idiosyncratic way. At best, it movements of the epicaudal appear to be correlated to making tight turns around obstacles or rotating in place, including maneuvers to position the coelacanth into the infamous “headstand” posture that baffled researchers for years. So the epicaudal could act as a sort of small rudder involved in fine-scale adjustments. However, given the relatively minuscule surface area presented by the epicaudal, it seems unlikely that it generates enough thrust force to contribute to locomotion (Fricke & Hissmann 1992; Hissmann & Fricke 1996).
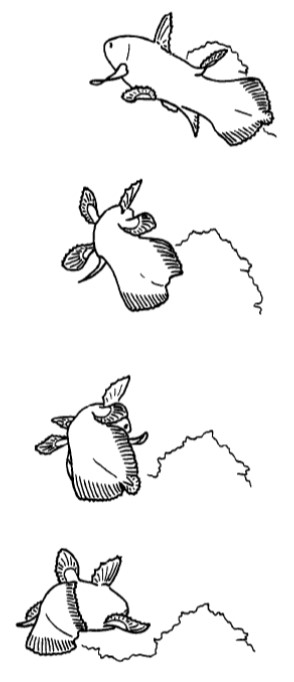
The coelacanth only employs its broad tail for fast starts and forward acceleration, which is usually not the preferred pace of life for this relaxed drifter. In this figure, a coelacanth maneuvering around an obstacle was observed flexing the epicaudal lobe towards the obstacle, perhaps acting as a fine-tuned rudder. But, for the most part, the epicaudal lobe seems to have a mind of its own. Image credit: Fricke & Hissmann 1992.
Movements of the epicaudal lobe have also been correlated to bringing the coelacanth into the bizarre headstand position. Researchers now believe the headstand is meant to bring electrosensory receptors on the face closer to the substrate, where tasty prey items may be located. Both this figure and the preceding one are tracings from video footage of live animals. Image credit: Fricke & Hissmann 1992.
There was one more observation about the epicaudal lobe in living coelacanths. The epicaudal had a tendency to be held at this 90º bent position while the fish was stationary. This lead Hissmann and Fricke to hypothesize a sensory function for the epicaudal. The pressure-sensitive lateral line of fish usually runs the length of the body and terminates at the base of the tail, right before the fin rays. In coelacanths, the well-developed lateral line extends to the very tip of the tail through the epicaudal. By kinking the epicaudal lobe perpendicular to the main axis of the body, it would be possible to pick up sensory cues about water movement not just hitting the sides of the body, but also in the fore and aft direction. Having spatial awareness of movement in relationship to water currents would appear to be especially adaptive for keeping the coelacanths positioned in the narrow little caves they favor as resting spots (Hissmann & Fricke 1996).
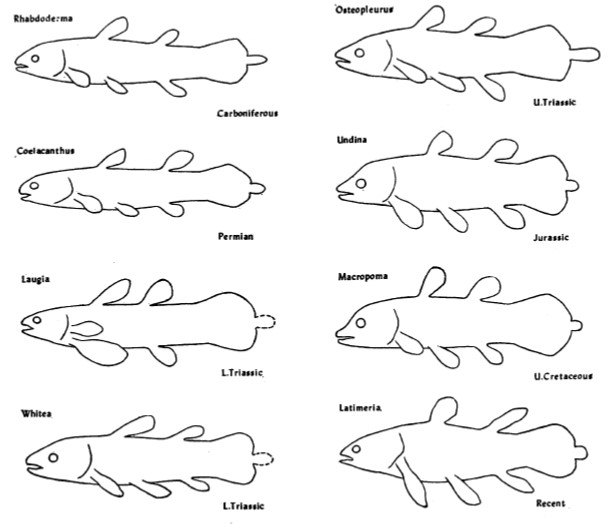
Body outlines of coelacanths from the Carboniferous period to the Late Cretaceous to the present day. Very little has changed over 300 million years. Or has it? Image credit: Schaeffer 1948.
Here’s a final curiosity from the fossil record. The coelacanth lineage is famous for having a generally unchanged body plan since its origins 300 million years ago, which led scientists to view them as slow evolving and essentially morphologically static (Forey 1998; Amemiya et al. 2013). And the strange little epicaudal lobe was there from the beginning. We do not know what function the epicaudal served over millions of years, but it quite certain that ancient coelacanths were not all just huddling in deep water caves. An exceptional example is the Early Triassic coelacanth from British Columbia, Canada: Rebellatrix divaricerca. Unlike the long line of coelacanths before and after it, R. divaricerca was slender and had a deeply forked tail, indicative of fast swimming in open water. Even so, a very distinct epicaudal was still present (Wendruff & Wilson 2012).
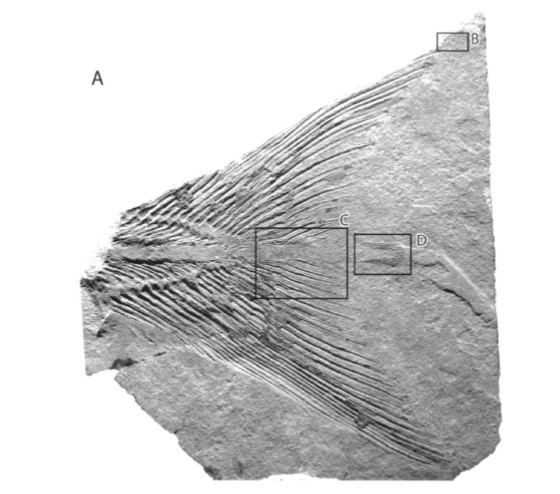
Fossil of the paratype for Rebellatrix divaricerca, an Early Triassic coelacanth with a highly unusual forked tail. The boxed area marked “D” shows the epicaudal lobe. Image credit: Wendruff & Wilson 2012.
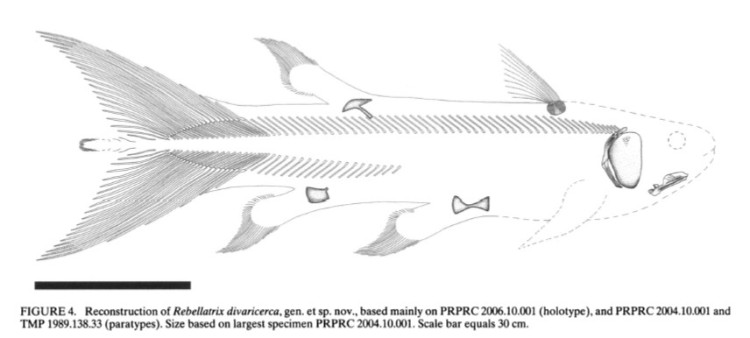
Reconstruction of the fast, sleek Rebellatrix divaricerca. Image credit: Wendruff & Wilson 2012.
Ultimately, the function of the epicaudal lobe remains unknown. Perhaps this is unsurprising. Between 1938 and 2014, less than 300 coelacanths have been captured and studied. Observing the animals in their natural environment usually necessitates the use of submersibles or remotely-operated vehicles (ROVs), though there is one population in Sodwana Bay, South Africa that lives within reach of SCUBA divers (Amemiya, Dorrington, & Meyer 2014).
What is particularly alarming is that the species of coelacanth discovered by Marjorie Courtenay-Latimer, named Latimeria chalumnae in her honor, is now listed as being Critically Endangered (Musick 2000). A second species, L. menadoensis, also serendipitously discovered in a fish market in Indonesia in 1997, is even less understood and listed as Vulnerable (Erdmann 2008; Amemiya, Dorrington, & Meyer 2014). The mystery of the coelacanth’s “puppy dog tail” endures, along with other fundamental questions about breeding biology, behavior, and ecology. There is so much left to learn. Coelacanths are also amongst our closest living fishy relatives. Let’s allow curiosity and kinship to keep this 300 million year old lineage going strong!

How can you not love this wonderful face? Image credit: Laurent Ballesta via National Geographic.
References
Amemiya, Chris T., R. Dorrington, & A. Meyer. 2014. “The Coelacanth and Its Genome.” Journal of Experimental Zoology (Mol. Dev. Evol.) 322 (6): 317 – 321.
Amemiya, Chris T. et al. 2013. “The African coelacanth genome provides insights into tetrapod evolution.” Nature 496: 311 – 316.
Erdmann, M. 2008. “Latimeria menadoensis.” The IUCN Red List of Threatened Species 2008. Accessed 4 September 2016.
Forey, Peter. 1998. History of the Coelacanth Fishes. Londong: Chapman & Hall.
Fricke, Hans & Karen Hissmann. 1992. “Locomotion, fin coordination and body form of the living coelacanth Latimeria chalumnae.” Environmental Biology of Fishes 34: 329 – 356.
Fricke, Hans, O. Reinicke, H. Hofer, & W. Nachtigall. 1987. “Locomotion of the coelacanth Latimeria chalumnae in its natural environment.” Nature 329: 331 – 333.
Hissmann, Karen & Hans Fricke. 1996. “Movements of the Epicaudal Fin in Coelacanths.” Copeia 1996 (3): 606 – 615.
Musick, J.A. 2000. “Latimeria chalumnae.” The IUCN Red List of Threatened Species 2000. Accessed 4 September 2016.
Schaeffer, Bobb. 1948. “A Study of Diplurus longicaudatus with Notes on the Body Form and Locomotion of the Coelacanthini.” American Museum Novitates 1378: 1 – 32.
Uyeno, Teruya. 1991. “Observations on locomotion and feeding of released coelacanths, Latimeria chalumnae.” Environmental Biology of Fishes 32: 267- 273.
Wendruff, Andrew J. & Mark V.H. Wilson. 2012. “A Fork-Tailed Coelacanth, Rebellatrix divaricerca, gen. et sp. nov. (Actinistia, Rebellatricidae, fam. nov.) from the Lower Triassic of Western Canada.” Journal of Vertebrate Paleontology 32 (3): 499 – 511.



