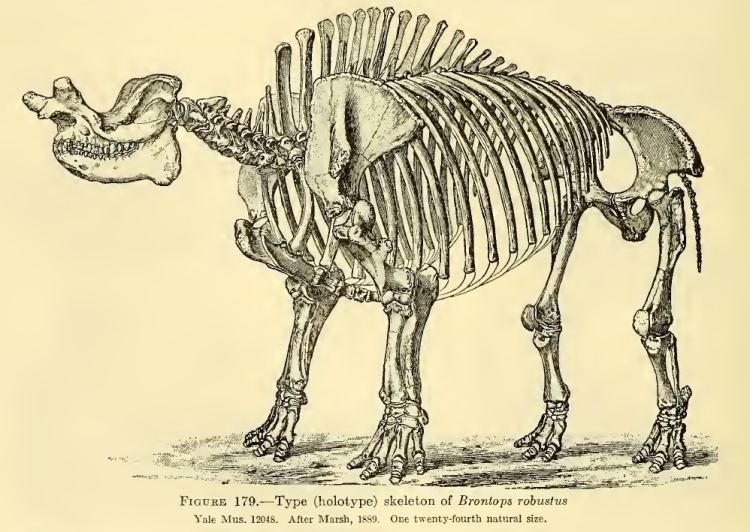
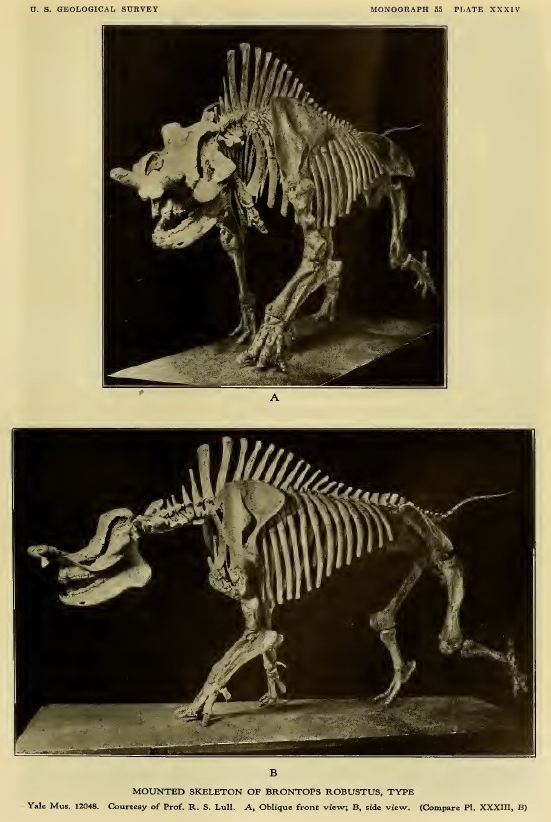
In 1930, English geologist John Parkinson described a species of stegosaur in less than savory terms:
The reptile. . . .belonged to the most uncouth group of all the stegosauria or armoured dinosaurs. . . . Stupidity and slowness seemed to be stamped on every bone of the beast.
One detail, common to the stegosaur type of dinosaur. . . is the expansion of the canal, which carries the great nerve (the neural cord) from the brain through the arch of the vertebrae, to the sacral or hip region, an expansion so enormous that the enclosed nerve matter exceeded in size that of the brain itself. . . .
In fact, the reptile had two brains. . . . [and the hinder one] looked after the functions of nutrition, digestion, and propagation, practically all that life’s daily routine required in a stegosaur.

Stegosaurus is a sizable beast, with the largest individuals of certain species growing to nearly 30 ft (9 meters) in length. In comparison to this enormous bulk, the head does appear ludicrously tiny in proportion. Image credit: Yale Peabody Museum / Curious Sengi.
Of all the dinosaurs known to us, Stegosaurus seems to be singled out for being particularly stupid. So stupid, in fact, that this iconic beast required a second brain in its hip to muddle through life. It is a strange snippet of information that continues to persist. . . . . so where did this idea come from?
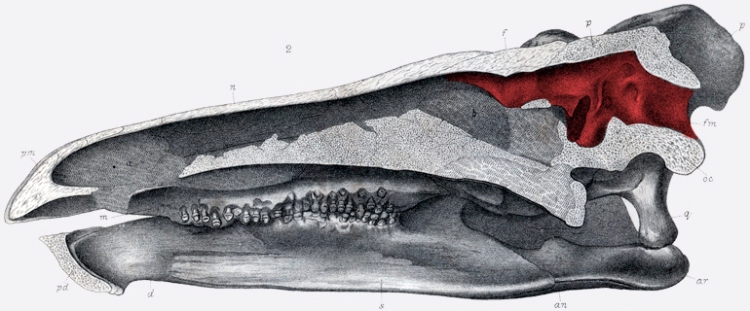
The cranial cavity that once contained the brain is colored red in this drawing of a cross-sectioned stegosaur skull. Marsh noted the general elongate shape of the brain, enlarged optic lobes suggesting the relative importance of vision in these animals, and small cerebral hemispheres. Image credit: public domain via Wikimedia Commons.

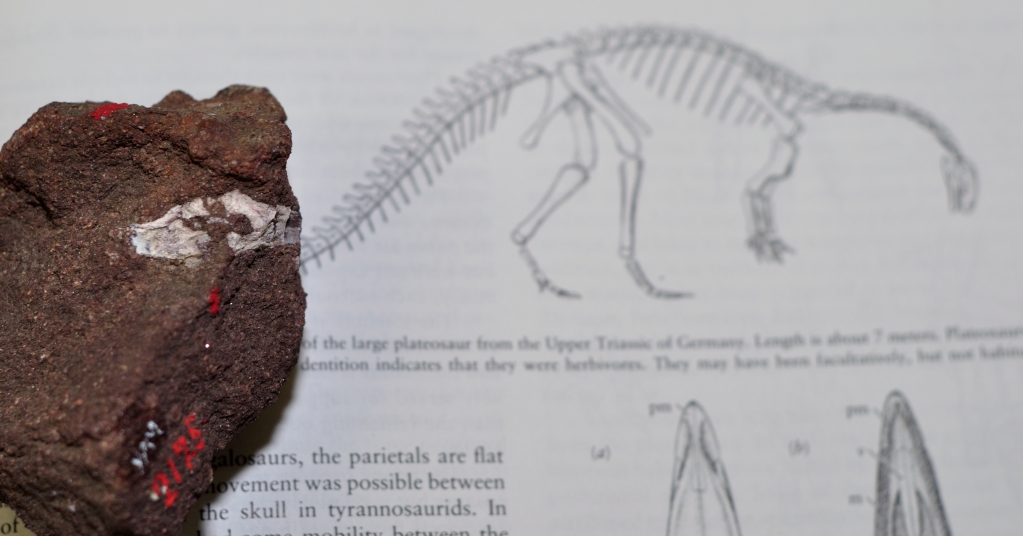
Original collection labels for some of the Stegosaurus fossil material collected for O.C. Marsh. Note the label on the right describing a natural brain cast, or endocast. Image credit: Yale Peabody Museum / Curious Sengi.
The first stegosaurs were discovered from Late Jurassic (~150 ma) rocks in Colorado by paleontologist O.C. Marsh (1831 – 1899). If not otherwise preoccupied with his role as co-villain in the infamous Bone Wars, Marsh ventured into new realms of inquiry including the study of endocasts, which are molds of internal, hollow spaces — in this case, spaces in the skull or vertebrae once occupied by the brain and other nervous system structures. Looking at these ancient brains not only gave a general sense of their size, but could also reveal finer details indicating heightened development of certain senses such as vision or olfaction. Marsh found at least one natural fossil endocast of a stegosaur brain. Based on some quick calculations, he remarked that if a stegosaur and a modern alligator were scaled to the same body size, the stegosaur brain would be 1/100th the size of the alligator’s. This led to the grim conclusion that: “Stegosaurus had thus one of the smallest brains of any known land vertebrate (Marsh 1896).”
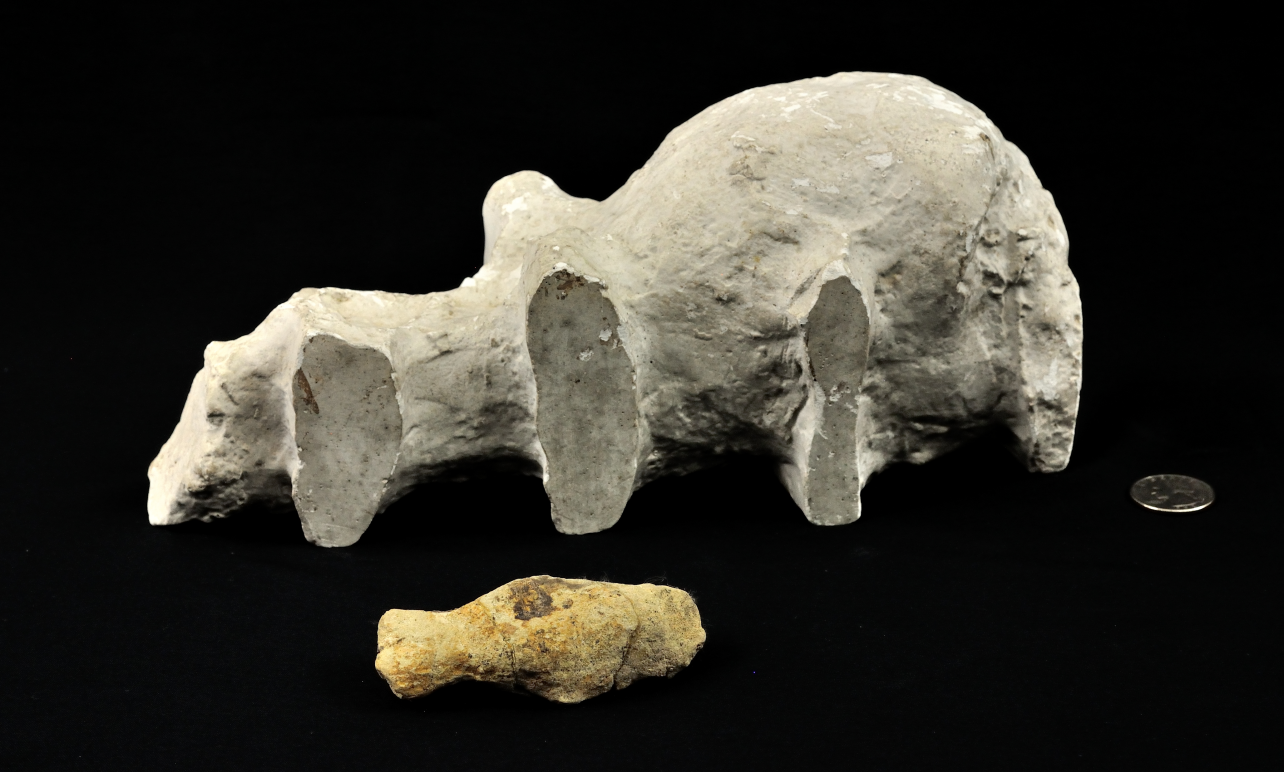
Sowing the seeds of suspicion: comparatively massive white plaster cast of the stegosaur sacral cavity in the background with a natural stone endocast of the brain cavity in the foreground. The plaster cast captures the enlargement of the spinal cord as it passed through the sacral (i.e., pelvic) vertebrae. Marsh believed the sacral cavity housed a secondary neural center that was an estimated 20 times larger than the brain. U.S. quarter for scale. Image credit: Yale Peabody Museum / Curious Sengi.
To make matters worse, Marsh made an artificial endocast in plaster of the sacral cavity, i.e., the hollow passage for the spinal cord in the vertebrae that are fused to the pelvis. This cavity was unusually large, more so than would be expected. While it was observed from a wide variety of vertebrates that the spinal cord bulks up where it sends off nerve branches to innervate the fore- and hindlimbs, the stegosaur’s enlarged neural cavity with its intervertebral bulges was exceptional. The sacral cavity was estimated to be at least 20 times the size of the brain.
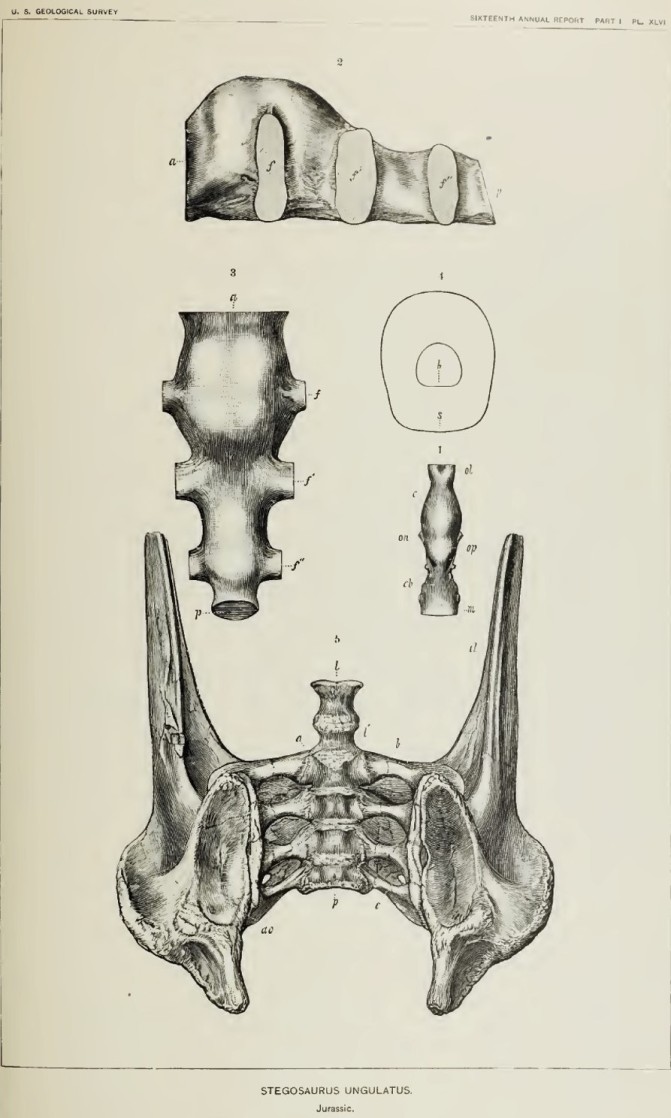
One of the plates used in Marsh’s description of Stegosaurus from the American West. The top figures (2 & 3) show views of the plaster cast made of the sacral cavity, complete with the sideways bulging into the intervertebral space (labeled f, f’, and f”). Figure 4 shows a schematic cross section of the relative size of the brain and sacral cavity. Image credit: Marsh 1896.
Given this vaguely brain-shaped hollow and, more interestingly, a particular pattern of growth in size between juveniles and adults, Marsh described the sacral cavity as a “posterior brain case” and “a posterior nervous center” — thus, the notion of a Stegosaurus butt brain was born.
Marsh seemed content with equating the presence of a secondary nerve center with a “. . . . posterior part that was dominant,” which one could imagine referred to the great weight borne upon the hindlegs and the coordination necessary to swing the stegosaur’s spiked tail against foolhardy predators or rivals. German scientists Branca and Waldeyer went so far as to ascribe a certain independence in function, affirming that this was indeed a proper “sacral brain” delegated to back-half duties: digestion and sex.

A set of stegosaur sacral vertebrate. The hollow through the middle shows the opening into the sacral cavity. This specimen was also collected for Marsh. Image credit: Yale Peabody Museum / Curious Sengi.
The role of intelligence — as interpreted through brain size alone — in the evolutionary succession of life on Earth was a particular obsession of the late 19th and early 20th century Western world. The discord between the gargantuan body size of the dinosaurs and their pathetically small brains captured the public imagination. It seemed like an obvious rationale for why dinosaurs went extinct.
More recent scientific studies have revisited the question of stegosaur brains and the results are much more nuanced.
Extrapolation from data collected from living reptiles showed that the vast majority of dinosaurs seemed to fall within the expected range of brain size to body mass proportions. While the stegosaur brain remained somewhat below the expected prediction, its size was consistent with large animals enjoying an undemanding “slow, herbivorous lifestyle (Buchholtz 2012).” Even if Stegosaurus was not catastrophically stupid, what about that “second brain”?

There was nothing bright going on the sacral cavity. Even though paleontologists no longer accept the idea of a secondary brain, what occupied this space along with the spinal cord remains inconclusive. Lateral view of sacral vertebrae with the internal cavity illuminated through the intervertebral spaces. Image credit: Yale Peabody Museum / Curious Sengi.
Paleontologists today emphatically reject the notion of a “sacral brain.” There are a number of hypotheses proposed for why stegosaurs possessed such enlarged sacral cavities, the most popular of which is that this space housed an agglomeration of tissue called a glycogen body. Glycogen bodies are only definitively found in the sacral cavities of modern birds and consist of carbohydrate-rich cells. While this structure has not been extensively studied, it is believed to provide metabolic support for the central nervous system, especially during the formation of myelin, a fatty layer of cells that insulate nerve fibers. As living descendants of the dinosaur lineage, it seemed plausible that the avian glycogen body is homologous to mysterious item occupying the stegosaur’s pelvis.
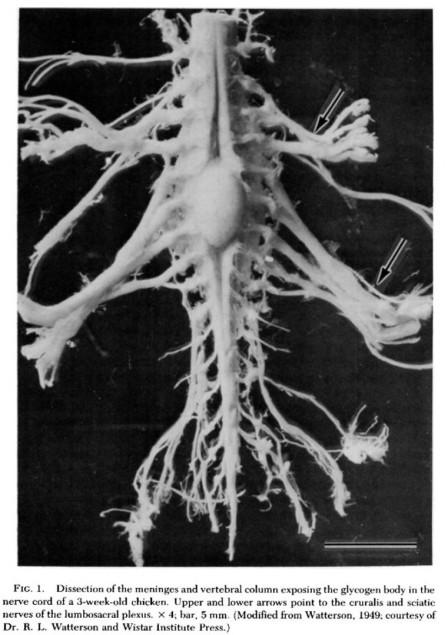
The avian glycogen body is an oval-shaped mass on the spinal cord. The function of this structure is not fully understood, but is probably involved in the metabolism of nervous system tissues. Image credit: De Gennaro 1982.
It should be noted that sacral enlargements for a putative glycogen body are found only in stegosaurs and another heavy herbivore, those long-necked titans: the sauropods. However, neither are stegosaurs and sauropods closely related to each other, nor are they closely related to birds. Dinosaurs that are in the direct line to modern birds, such as coelurosaurs, lack any kind of enlargement of the sacral cavity. In the end, the question of why Stegosaurus had such a large hollow in its hip remains a dissatisfying enigma. But, if anything, it definitely was not a brain with an independent agency as Branca and Waldeyer believed.
Stegosaurs were certainly not very bright, but they did quite well for several million years. And that is considerably longer than the approximately 200,000 year history of anatomically modern humans and the current future we have set upon.
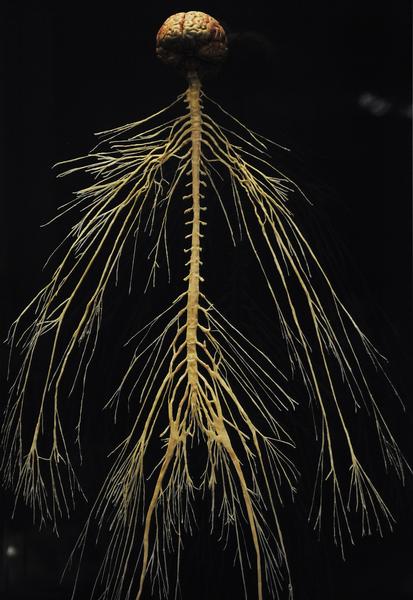
Plastinated specimen of the human nervous system. Note the thickening of the spinal cord where nerves for the arms and legs branch off. This is the usual pattern in vertebrates. From the “Body Worlds” 2010 exhibit at the Denver Museum of Nature and Science. Image Credit: Cyrus McCrimmon / The Denver Post.
References
Benzo, C.A. & L.D. De Gennaro. 1983. “An Hypothesis of Function for the Avian Glycogen Body: A Novel Role for Glycogen in the Central Nervous System.” Medical Hypotheses 10: 69 – 76.
Buchholtz, Emily. 2012. “Dinosaur Paleoneurology.” In The Complete Dinosaur. 2nd edition. M.K. Brett-Surman, T.R. Holtz, Jr., & J.O. Farlow, editors. Indiana University Press. Pp. 191 – 208.
De Gennaro, Louis D. 1982. “Chapter 6: The Glycogen Body.” In Avian Biology, Vol. VI. D.S. Farner, J.R. King, & K.C. Parkes, editors. Academic Press. Pp. 341 – 372.
Galton, Peter M. & P. Upchurch. “Chapter Sixteen: Stegosauria.” In The Dinosauria, 2nd edition. 2004. D.B. Weishampel, P. Dodson, & H. Osmólska, eds. University of California Press. Pp. 343 – 362.
Griffin, Emily. 1990. “Gross Spinal Anatomy and Limb Use in Living and Fossil Reptiles.” Paleobiology 16 (4): 448 – 458.
Lull, Richard Swann. 1917. “On the Functions of the ‘Sacral Brain’ in Dinosaurs.” American Journal of Science 44: 471 – 477.
Marsh, O.C. 1896. “The Dinosaurs of North America.” Sixteenth Annual Report of the U.S. Geological Survey, Pt. I: 133 – 244.
Parkinson, John. 1930. The Dinosaur in East Africa: An Account of the Giant Reptile Beds of Tendaguru, Tanganyika Territory. H.F. & G. Witherby.